

For instance, chemical energy is converted to kinetic energy when a stick of dynamite explodes. The reaction occurs in the following manner. This law, first proposed and tested by Émilie du Châtelet, means that energy can neither be created nor destroyed rather, it can only be transformed or transferred from one form to another. When 100g of HgO is heated, it decomposes into two compounds: 92.6g of Hg and 7.4 g of \(O_2\) Hence, the law of conservation of mass is proved.Įxample 2. One will notice that the physical property of ice changes into water upon heat, but the mass is retained.
\(Ice \rightarrow Heat \rightarrow Water\) Let us understand the law of conservation of mass with the following examplesĮxample 1. V = flow velocity Examples of Law of Conservation of Mass The formula for law of conservation of mass is given by, The mass of reactants is equal to the mass of products for any low energy thermodynamic process.

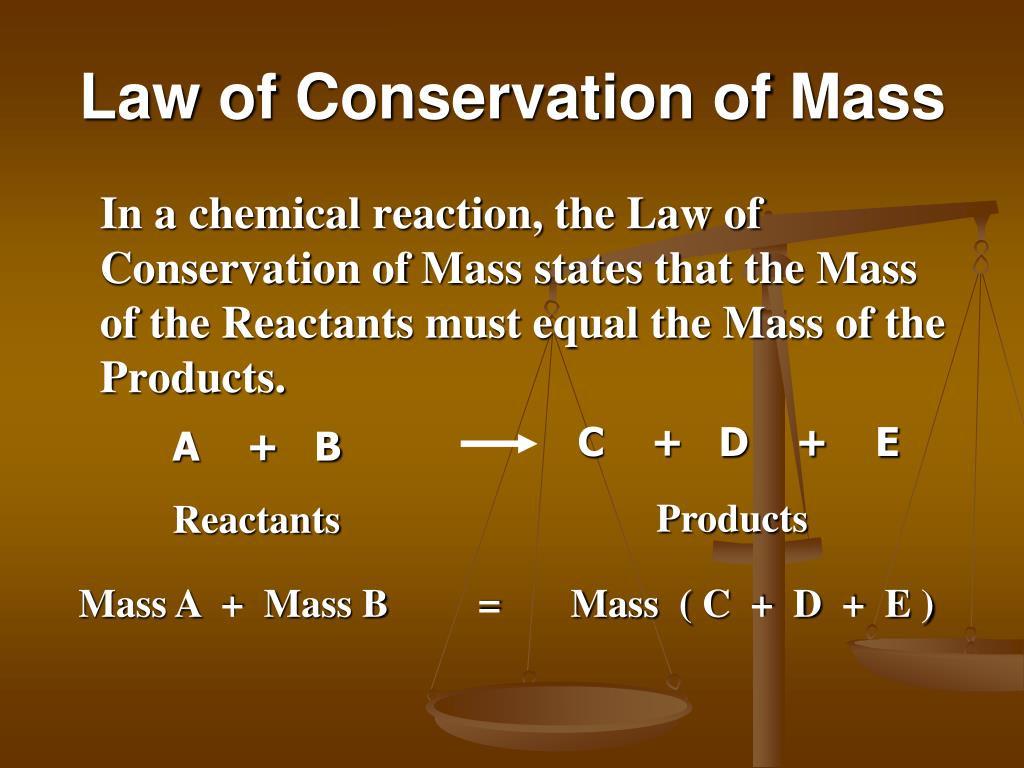
Mass of calcium carbonate = mass of calcium oxide + mass of calcium dioxide The Law of Conservation of Mass states that “mass can neither be created nor be destroyed in a reaction.” The total mass shall always be retained from the start of the reaction till the end.Īccording to this law, matter cannot be created nor destroyed, also known as the law of indestructibility of matter.įrom the following Law of conservation of mass examples let us understand the concept for more clarity.įor example, 10 grams of calcium carbonate produces 5.6 grams of calcium oxide and 4.4 grams of calcium dioxide. Dive into this Physics articleto learn about law of conservation of mass with examples.


 0 kommentar(er)
0 kommentar(er)
